Research themes
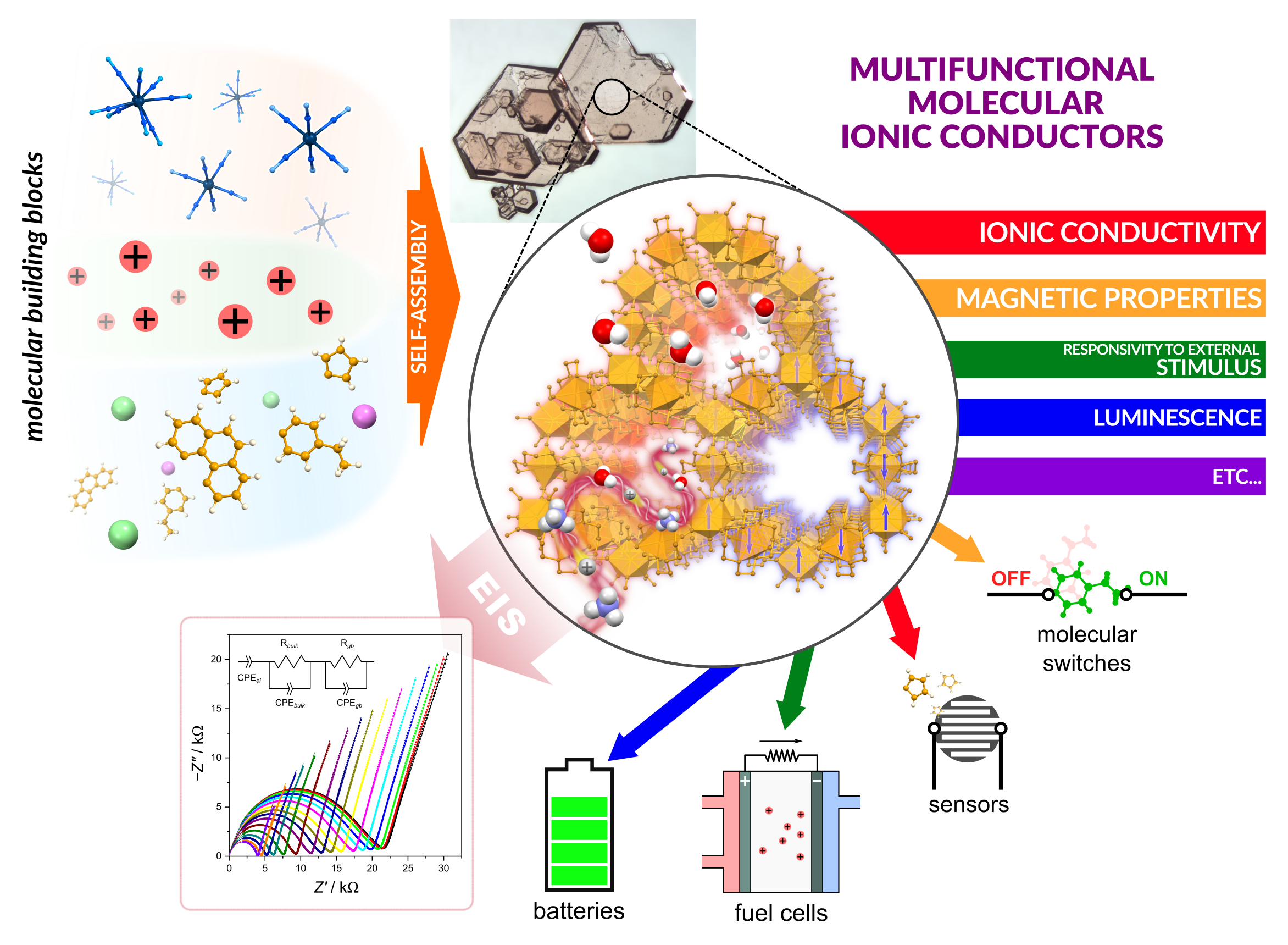
Every year, energy consumption in the world increases, which constitutes a significant civilization challenge. Hence, the key is to search for new, efficient, and clean sources of energy and methods of its storage. Potential solutions are fuel cells (e.g. hydrogen) and batteries. In both cases, solid ionic conductors which can be used as solid electrolytes in batteries or ion exchange membranes in fuel cells become useful. This is the main motivation behind our research into new ionic conductors.
In our research, we focus on the implementation of ionic conductivity into molecular cyanido-bridged coordination systems, which exhibit plenty of functionalities, such as magnetic order, Single-Molecule Magnet properties, luminescence, and sensitivity to chemical and physical stimuli. The purpose of this combination of functionality is to obtain materials that can be used not only as solid electrolytes or ion exchange membranes but also as molecular switches and sensors for molecular electronics.
The advantage of molecular materials, such as cyanido-bridged coordination systems, is the possibility of rationally designing their functionality at their molecular level by appropriate selection of the molecular components. Important components of conductive materials are charge carriers, which in the case of ionic conductors are mobile ions, usually cations. Based on the type of cationic species, ionic conductors can be divided into proton conductors (charge carriers are H+ cations) or non-protonic conductors (Li+, Na+, K+, etc.). In our research, we develop synthetic strategies which allow for the introduction of charge carriers into CN−-bridged coordination systems with simultaneous design of their structures to ensure appropriate paths for charge transport. The ionic conductivity in the obtained materials is tested with the use of electrochemical impedance spectroscopy (EIS).
Representative publications
- M. Reczyński, B. Nowicka, C. Näther, M. Kozieł, K. Nakabayashi, S. Ohkoshi, B. Sieklucka, "Dehydration-Triggered Charge Transfer and High Proton Conductivity in (H3O)[NiIII(cyclam)][MII(CN)6] (M = Ru, Os) Cyanide-Bridged Chains". Inorg. Chem., 2018, 57, 13415-13422.
- M. Reczyński, M. Heczko, M. Kozieł, S. Ohkoshi, B. Sieklucka, B. Nowicka, "Proton-Conducting Humidity-Sensitive NiII-NbIV Magnetic Coordination Network", Inorg. Chem., 2019, 58, 15812-15823.
- J. Wang, J. J. Zakrzewski, M. Heczko, M. Zychowicz, K. Nakagawa, K. Nakabayashi, B. Sieklucka, S. Chorazy, S. Ohkoshi "Proton Conductive Luminescent Thermometer Based on Near-Infrared Emissive {YbCo2} Molecular Nanomagnets", J. Am. Chem. Soc., 2020, 142, 3970-3979.
Cooperation opportunities
Those of you who are interested in performing electrochemical impedance spectroscopy studies are kindly asked to contact dr. Mateusz Reczyński (mateusz.reczynski@uj.edu.pl). Students who would be interested in the development of some of the research tasks in the SONATA 16 project ‘Ionic conductors based on cyanido coordination architectures: design and functionalization’ should contact dr. Reczyński (PI) as well.

